What is Molecule, Element and Compound?
Molecule and Molecule of Elements
The learning about molecules forms the reason for further ideas in science. Much the same as blocks structure an assortment of structures, iotas lead to the arrangement of molecules and aggravates that are a piece of issue around us. Give us a chance to attempt to get a reasonable thought regarding the diverse arrangements of iotas.
Element
You can consider a component ass the key substance which comprises of a solitary kind of atom. Moreover, elements involve littler particles and can either be engineered or man-made. The course of action of elements in the occasional table is moved toward the premise of the quantity of protons included in an expanding request.
Additionally, when molecules are diversely organized in a component holding the quantity of protons, one would get distinctive adaptations of that component. Model: both precious stone and graphite are elements of carbon; be that as it may, they appear to be very unique from each other.
Molecule
A molecule can be named as the littlest unit related with a synthetic compound. It conveys a similar concoction properties of that specific compound. Since molecules are worked out of iotas which are mutually held by means of compound bonds, there are odds of huge fluctuation as far as size and unpredictability.
For instance, oxygen holds a sub-atomic recipe O2. Do we think about it as a molecule or compound? At whatever point at least two particles of a similar component combine, we label them as molecules. Henceforth, O2 remains as an oxygen molecule.
Compound
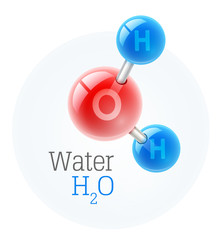
Keep in mind that, the concoction recipe for water is H2O. In this note, water is produced using 2 hydrogen atoms and 1 oxygen particle. Two distinct elements consolidate, bringing forth another substance called as water. Thus, those substances made by the merger of at least two kinds of elements are Mixes.
Atoms present in a compound are artificially associated and subsequently can't be isolated effectively. So also, the compound recipe for carbon dioxide is CO2, and it is shaped utilizing two elements, specifically carbon and oxygen. Some more instances of mixes incorporate table salt (NaCl), chalk (CaCO3) and water (H2O).
Distinction Between a Compound and Molecule
To be exact, a compound is fundamentally a substance which is created utilizing at least two distinct elements. Table salt (NaCl), Water (H2O), carbon dioxide (CO2) and so forth., are a few instances of mixes. These are called as mixes since every one of them contain more than one sort of component.
Then again, nitrogen gas (N2), just as buck minster fullerene (C60), can't be delegated mixes. This is on the grounds that they just contain a solitary sort of component. For exact information, something which is a molecule doesn't rely upon the sort of bond made when particles consolidate.
All things considered, electrons are allowed to be shared between particles or electrons can be completely expelled from a solitary iota and exchanged to another. Keep a note that, molecules contain atomic bonds. N2 can be viewed as a molecule since the bond framed between the nitrogen iotas portrays the atomic bond.
Water (H2O) is a sub-atomic compound since it is a substance produced using more than one sort of component that is held together by sub-atomic bonds. Further, Salt (NaCl) takes after an ionic compound since it is produced using more than one kind of component which is held together by ionic bonds.









No comments