What is Chemical Formulae?
A chemical recipe is characterized as an accumulation of chemical image that is utilized to demonstrate the components present in any compound and its extent. Before we invasion into the zone of writing a chemical recipe, we should familiarize ourselves with the essentials of chemical formulae. Give us a chance to continue further!
What is A Particle?
Have you seen how a house is made, step by step? Everything begins with laying the principal block which bit by bit graduates into an undeniable divider. In the event that this divider is a chemical compound, at that point the block is the iota. A molecule is the littlest resolute molecule in any chemical condition.
While they might be the littlest unit of a chemical aggravate, their significance is essentially similar to that of the block used to manufacture the house. Without it, there will be no house. Correspondingly, our whole universe is comprised of iotas.
Each component in nature is implied with a chemical name, a chemical valency and an image which is utilized to allude to this component while making chemical conditions. This image signifies a solitary iota. In other words, when we allude to Aluminum as Al, it alludes to a solitary particle of Aluminum.
The principal researcher to utilize images for chemical components was Dalton. He was the person who utilized the images of the components in a predetermined amount, i.e one molecule of the component. Later on, Berzilius proposed that we could utilize a couple of letters of the component to frame its image.
At first, images for the name of components were gotten from the spot they began from. So copper was taken from Cyprus, while gold was implied yellow thus it was signified in like manner. In any case, today the Global Association of Unadulterated and Connected Science favors the component names.
Customarily, the primary letter of the name of the component in capitalized pursued continuously letter which is in lower case is composed as the image of the component. Now and again you may see that the letter sets indicating the image of the component does not coordinate the name of the component. This happens when the name of the component is written in English however the image is gotten from its name in Latin, Greek or German.
For instance, the chemical image for Potassium is K. This 'K' is gotten from Kalium. Iron is indicated by 'Fe' which originates from the Latin word "Ferrum". H for Hydrogen, Co for Cobalt. This guarantees every component has an extraordinary image.
What is A Nuclear Mass?
Dalton in his Nuclear hypothesis further conjectured that every component comprised of a nuclear mass. This hypothesis was generally acknowledged as it clarified the law of steady extents and the researchers were urged to gauge the nuclear mass of the components. In any case, it was before long understood that deciding the mass of an iota was not a simple exercise.
Hence, researchers built up a method for deciding nuclear load by framing mixes utilizing the law of chemical blends. In 1961 a standard unit of Carbon iota was resolved to be 12 isotopes (Get familiar with Isotopes and Isobars here). It was suggested that one nuclear mass unit was 1/twelfth of the mass of the mass of a solitary carbon particle. In view of this general masses of different components were additionally decided.
For instance, oxygen has a nuclear mass of 16. Sodium has a nuclear mass of 23 and in like manner different components additionally got their nuclear masses.
Get familiar with Nuclear Number here.
Chemical Recipe
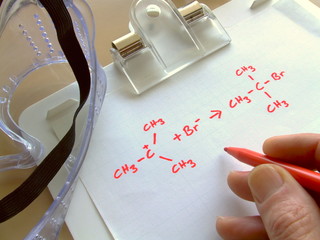
As officially expressed, a chemical recipe is an emblematic articulation implying the quantity of iotas present in an atomic substance. We decide the kind of particle by alluding to its image, so for Hydrogen, we will utilize H. The quantity of iotas is dictated by the subscript joined to the image. So a chemical recipe of water, i.e H2O has two molecules of hydrogen and a solitary particle of oxygen.
Ventures for writing a chemical equation
Stage 1: First, you need to choose the kind of the bond.
On the off chance that the prefixes are utilized, at that point it is a covalent bond. In the event that there are no prefixes, it is an ionic bond. After that is chosen move to Step number 2.
Stage 2: Presently, record the image of the polyatomic particle or the component.
Stage 3: Presently, if the prefix was utilized, you'll need to include a subscript. You'll additionally need to add a subscript so as to adjust the charge.







No comments